Abstract
Many drugs, or their antecedents, were discovered through observation of their effects on normal or disease physiology. For the past generation, this phenotypic drug discovery approach has been largely supplanted by the powerful but reductionist approach of modulating specific molecular targets of interest. Nevertheless, modern phenotypic drug discovery, which combines the original concept with modern tools and strategies, has re-emerged over the past decade to systematically pursue drug discovery based on therapeutic effects in realistic disease models. Here, we discuss recent successes with this approach, as well as consider ongoing challenges and approaches to address them. We also explore how innovation in this area may fuel the next generation of successful projects.
Introduction
Historically, new medicines were discovered through observation of their therapeutic effect on disease phenotypes, either directly in humans as part of traditional medicine or in models of disease. With the advent of the molecular biology revolution in the 1980s and the sequencing of the human genome in 2001, the focus shifted to specific molecular targets. Since 2011, however, phenotypic drug discovery (PDD) has had a resurgence following the surprising observation that the majority of the first-in-class drugs approved by the US Food and Drug Administration (FDA) between 1999 and 2008 were discovered empirically without a drug target hypothesis1. The modern version of this legacy strategy is defined by its focus on the modulation of a disease phenotype or biomarker rather than a pre-specified target to provide a therapeutic benefit2. Ten years in, PDD is maturing as a field, serving as an accepted discovery modality in both academia and the pharmaceutical industry as opposed to a transient fad. This continued interest is rooted in notable successes in the past decade, including ivacaftor and lumacaftor for cystic fibrosis, risdiplam and branaplam for spinal muscular atrophy (SMA), SEP-363856 for schizophrenia, KAF156 for malaria and crisaborole for atopic dermatitis.
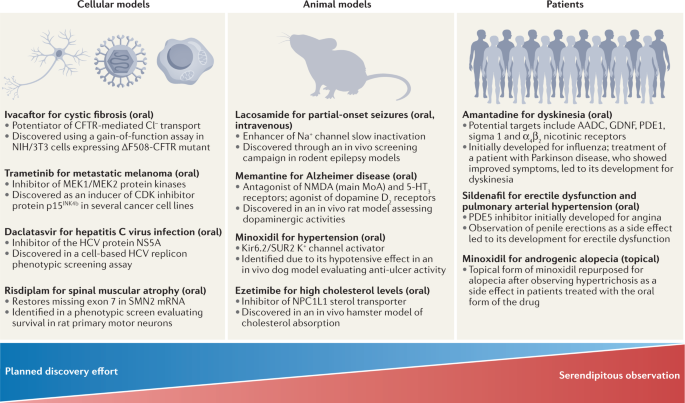
This is not to say that PDD approaches are a magic bullet to address issues with pharmaceutical industry productivity; the pros/cons of phenotypic screening need to be carefully balanced against molecular approaches for validated targets3. Although PDD has been successful, many historical examples used highly complex disease systems (in vivo models and even humans) rather than cell-based screens and/or were the result of serendipitous discoveries4 (Fig. 1), and complex models are only now regaining prominence. Drug repurposing provides a compelling example of this state of affairs, with well-known examples of repurposed drugs based on serendipitous clinical observations (for example, sildenafil, minoxidil, thalidomide and amantadine) on the one hand, and a lack of approved repurposed drugs stemming from pre-planned screens of clinical compound collections on the other5. This raises the critical question of how best to prospectively approach the discovery of novel drugs using phenotypic screening.
Examples of drugs originating from phenotypic drug discovery (PDD), also illustrating the contribution of planned discovery and serendipity. Approved drugs are listed based on the type of original phenotypic assay that first connected the compound series or the drug itself to the disease. All examples originating from cellular screens were the outcome of planned efforts, whereas all examples originating from studies in patients were based on unexpected clinical side effects that led to compound repurposing. References: ivacaftor13,14; daclatasvir90; risdiplam126; trametinib218; lacosamide78; memantine77; minoxidil76,219; ezetimibe220; amantadine221; sildenafil222. CFTR, cystic fibrosis transmembrane conductance regulator; HCV, hepatitis C virus; MoA, mechanism of action; SMN, survival of motor neuron.
In addition to a renewed appreciation for the complexities of physiology and pharmacology, PDD challenges our assumptions about what is druggable (with unusual targets and mechanisms of action (MoAs), including required polypharmacology to drive efficacy) and what is a drug (with unexpected compound properties being allowed). Whereas important hurdles remain related to target identification, steps for safety de-risking and the mapping of a clinical path for drug candidates originating from phenotypic screening, exciting opportunities are emerging for the application of functional genomics, machine learning/artificial intelligence and improved disease models.
Given the major differences between PDD and target-based drug discovery (TDD) and the new technologies that can now be brought to bear on PDD programmes, the field is evolving at a rapid pace, with a need to establish and share best practices across industry and academia3,6,7,8,9. Although technical and cultural hurdles remain, the renewed utilization of PDD has started to change the manner in which we conceptualize drug discovery and has proven to be an important testing ground for technical innovations in the life sciences. In this Perspective, we highlight our collective thoughts on how PDD has shaped concepts related to drug discovery and conclude with a discussion of the challenges ahead for maximizing the effectiveness of PDD.
Drug discovery concepts shaped by PDD
Expansion of ‘druggable’ target space
The main driver for PDD stems from the disproportionate number of first-in-class medicines derived from this approach1. In contrast to TDD, which is based on an established causal relationship between a molecular target and a disease state, PDD relies on chemical interrogation of a disease-relevant biological system in a molecular target-agnostic fashion. This empirical, biology-first strategy provides tool molecules to link therapeutic biology to previously unknown signalling pathways, molecular mechanisms and drug targets, as highlighted in the following four examples.
The first example is therapies for hepatitis C, a liver disease caused by the hepatitis C virus (HCV), which infects 3% of the population and is estimated to cause 300,000 deaths worldwide each year10. In the past decade, the treatment of HCV has been revolutionized by the development of combinations of orally available direct-acting antivirals that inhibit HCV replication, and clear the virus in >90% of infected patients. Modulators of the HCV protein NS5A such as daclatasvir are a key component of these direct-acting antiviral combinations. The importance of NS5A, which is essential for HCV replication but has no known enzymatic activity, as well as its small-molecule modulators, were initially discovered using a HCV replicon phenotypic screen11.
Another example is therapies for cystic fibrosis, a progressive and frequently fatal genetic disease caused by various mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that decrease CFTR function or interrupt CFTR intracellular folding and plasma membrane insertion12. Target-agnostic compound screens using cell lines expressing wild-type or disease-associated CFTR variants identified compound classes that improved CFTR channel gating properties (potentiators such as ivacaftor), as well as compounds with an unexpected MoA: enhancing the folding and plasma membrane insertion of CFTR (correctors such as lumacaftor, tezacaftor and elexacaftor)13,14. Notably, a combination of elexacaftor, tezacaftor and ivacaftor that addresses 90% of patients with cystic fibrosis was approved in 2019 (ref.15).
Third, inspired by observations that thalidomide effectively treated leprosy, modulated multiple anti-inflammatory cytokines, inhibited angiogenesis and showed activity in multiple myeloma16, the optimized analogue lenalidomide gained FDA approval for several blood cancer indications and has been highly successful (sales of more than US $12 billion in 2020)17,18,19. Significantly, the unprecedented molecular target and MoA of lenalidomide were only elucidated several years post-approval. Lenalidomide binds to the E3 ubiquitin ligase Cereblon and redirects its substrate selectivity to promote the ubiquitination and subsequent degradation of target proteins including the transcription factors IKZF1 and IKZF3 (ref.20). Furthermore, this novel MoA is now being intensively explored in the development of further targeted protein degraders, dubbed ‘bifunctional molecular glues’21.
Last, type 1 SMA is a rare neuromuscular disease with 95% mortality by 18 months of age. SMA is caused by loss-of-function mutations in the SMN1 gene, which encodes a protein known as survival of motor neuron (SMN) that is involved in the formation and maintenance of neuromuscular junctions. Humans also have a very closely related SMN2 gene, but a mutation that affects its splicing leads to exclusion of exon 7 and the production of an unstable shorter SMN variant. Phenotypic screens by two research groups independently identified small molecules that modulate SMN2 pre-mRNA splicing and increase levels of full-length SMN protein22,23. Both compounds work by engaging two sites at the SMN2 exon 7 and stabilizing the U1 small nuclear ribonucleoprotein particle (snRNP) complex22,24,25, an unprecedented drug target and MoA. One such compound, risdiplam, was approved by the FDA in 2020 as the first oral disease-modifying therapy for SMA.
Table 1 presents further recent examples of approved or clinical-stage compounds originating from phenotypic screens, including those for which the affected cellular processes are well defined but the specific entity that binds the compound is a multicomponent ‘cellular machine’ — a poorly defined molecular target. Taken together, these examples demonstrate how phenotypic strategies have expanded the ‘druggable target space’ to include unexpected cellular processes (pre-mRNA splicing, as well as target protein folding, trafficking, translation and degradation) and novel MoAs for traditional target classes (pseudo-kinase domain inhibition, allosteric kinase activation and masked covalent warheads) and have revealed new classes of drug targets (for example, bromodomains). They suggest that phenotypic strategies should be considered when no attractive target is known to modulate the pathway or disease phenotype of interest and/or the project goal is to obtain a first-in-class drug with a differentiated MoA.
Polypharmacology re-examined
With no restrictions in the available chemical and biological space other than those defined by the compound library and the disease model systems, phenotypic screening offers the opportunity to identify molecules engaging multiple targets, in what is known as polypharmacology26,27. In this scenario, the intended effect of a compound depends on a combination of targets (on-targets); however, these are not necessarily its full target signature, which may include targets not required for activity (off-targets).
In the quest for ever-more selective drugs, polypharmacology has been traditionally associated with poorly optimized compounds prone to potential side effects due to the difficulty in tracking all of the biological functions represented by off-targets. However, at therapeutically relevant concentrations, most, if not all, approved drugs are known to interact with multiple targets that often underlie side effects but can also contribute to clinical efficacy28,