Detail
转自:https://www.nejm.org/doi/full/10.1056/NEJMoa2209870
Jim Jones, M.D., Pharm.D., Darin J. Correll, M.D., Sandra M. Lechner, Ph.D., Ina Jazic, Ph.D., Xiaopeng Miao, Ph.D., David Shaw, Ph.D., Christopher Simard, M.D., Jeremiah D. Osteen, Ph.D., Brian Hare, Ph.D., Alina Beaton, M.D., Todd Bertoch, M.D., Asokumar Buvanendran, M.D., et al., for the VX21-548-101 and VX21-548-102 Trial Groups*
Abstract
BACKGROUND
The NaV1.8 voltage-gated sodium channel, expressed in peripheral nociceptive neurons, plays a role in transmitting nociceptive signals. The effect of VX-548, an oral, highly selective inhibitor of NaV1.8, on control of acute pain is being studied.
METHODS
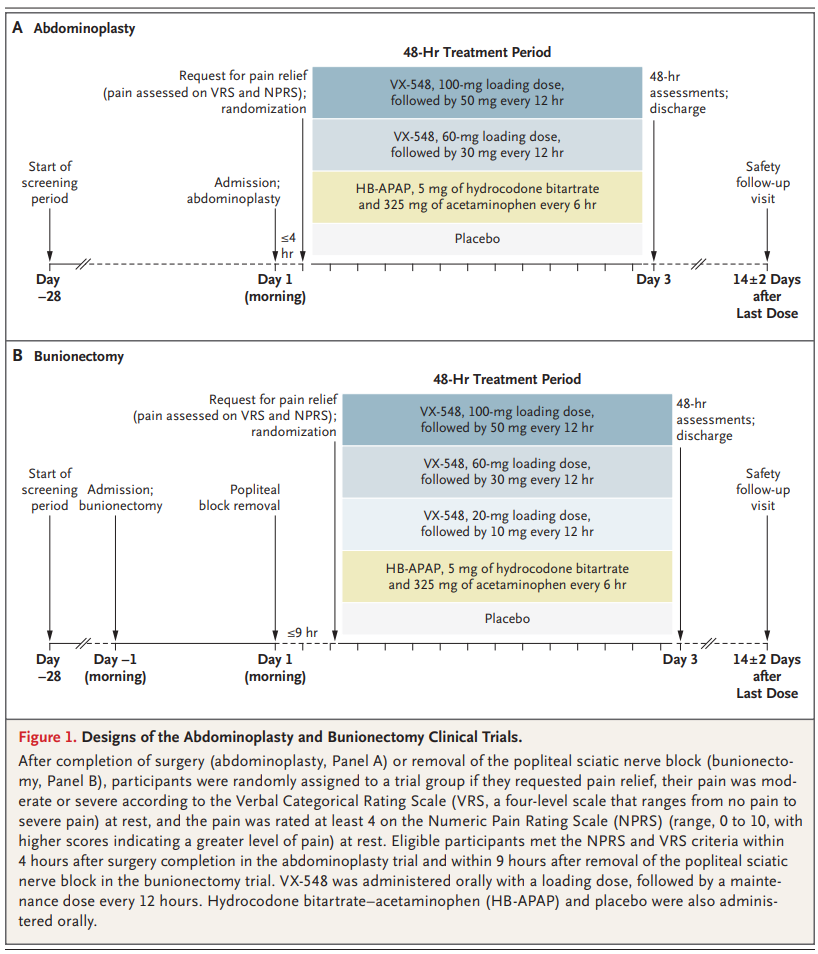
After establishing the selectivity of VX-548 for NaV1.8 inhibition in vitro, we conducted two phase 2 trials involving participants with acute pain after abdominoplasty or bunionectomy. In the abdominoplasty trial, participants were randomly assigned in a 1:1:1:1 ratio to receive one of the following over a 48-hour period: a 100-mg oral loading dose of VX-548, followed by a 50-mg maintenance dose every 12 hours (the high-dose group); a 60-mg loading dose of VX-548, followed by a 30-mg maintenance dose every 12 hours (the middle-dose group); hydrocodone bitartrate–acetaminophen (5 mg of hydrocodone bitartrate and 325 mg of acetaminophen every 6 hours); or oral placebo every 6 hours. In the bunionectomy trial, participants were randomly assigned in a 2:2:1:2:2 ratio to receive one of the following over a 48-hour treatment period: oral high-dose VX-548; middle-dose VX-548; low-dose VX-548 (a 20-mg loading dose, followed by a 10-mg maintenance dose every 12 hours); oral hydrocodone bitartrate–acetaminophen (5 mg of hydrocodone bitartrate and 325 mg of acetaminophen every 6 hours); or oral placebo every 6 hours. The primary end point was the time-weighted sum of the pain-intensity difference (SPID) over the 48-hour period (SPID48), a measure derived from the score on the Numeric Pain Rating Scale (range, 0 to 10; higher scores indicate greater pain) at 19 time points after the first dose of VX-548 or placebo. The main analysis compared each dose of VX-548 with placebo.
RESULTS
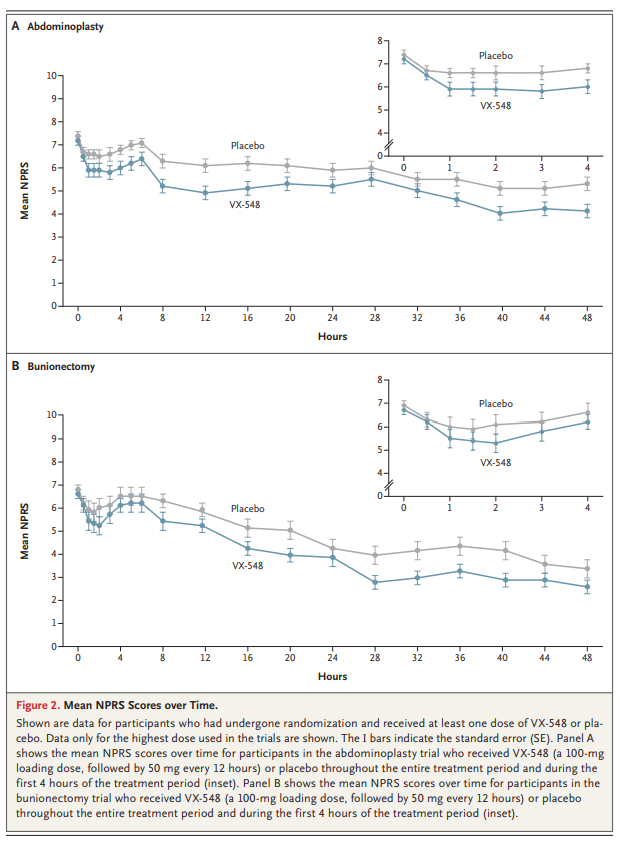
A total of 303 participants were enrolled in the abdominoplasty trial and 274 in the bunionectomy trial. The least-squares mean difference between the high-dose VX-548 and placebo groups in the time-weighted SPID48 was 37.8 (95% confidence interval [CI], 9.2 to 66.4) after abdominoplasty and 36.8 (95% CI, 4.6 to 69.0) after bunionectomy. In both trials, participants who received lower doses of VX-548 had results similar to those with placebo. Headache and constipation were common adverse events with VX-548.
CONCLUSIONS
As compared with placebo, VX-548 at the highest dose, but not at lower doses, reduced acute pain over a period of 48 hours after abdominoplasty or bunionectomy. VX-548 was associated with adverse events that were mild to moderate in severity. (Funded by Vertex Pharmaceuticals; VX21-548-101 and VX21-548-102 ClinicalTrials.gov numbers, NCT04977336. opens in new tab and NCT05034952. opens in new tab.)
Acute pain, a pervasive cause of suffering, has major implications for patients and public health.1,2 Opioids targeting central mechanisms involved in the perception of pain are often used for treatment of acute pain; however, their use is limited by safety concerns and the potential for misuse and addiction.3-6Nonopioid treatments for pain include nonselective sodium-channel inhibitors (e.g., lidocaine, mexiletine, and carbamazepine), nonsteroidal anti-inflammatory drugs (NSAIDs), and acetaminophen. Most approved analgesic drugs either act on the opioid-receptor system or are NSAIDs.7,8The voltage-gated sodium channel NaV1.8 is a therapeutic target for pain because of its role in transmitting nociceptive signals and its selective expression in peripheral nociceptive neurons of the dorsal-root ganglia.9-12 NaV1.8 is a sodium ion channel subtype that in humans is encoded by SCN10A. Our understanding of the role of NaV1.8 in pain transmission is supported by its function in normal sensory physiologic response,10-14 pathologic states arising from mutations in SCN10A,15,16animal models,17-20 and pharmacologic effects of NaV1.8-modulating agents.21,22 We hypothesized that selective inhibition of NaV1.8 would provide effective pain relief without the risks associated with opioid treatments. We describe the preclinical characterization of VX-548, an oral, highly selective inhibitor of NaV1.8, and we report the results of two Phase 2 trials evaluating the efficacy and safety of VX-548 in persons who had acute pain after abdominoplasty or bunionectomy.
转自:https://www.nejm.org/doi/full/10.1056/NEJMe2305480
Mark S. Wallace, M.D.
The use of opioid medications for pain management after surgery is a leading antecedent to persistent opioid use.1 The negative effects of these agents underscore the need for more nonopioid alternatives to treat postoperative pain; however, years of research and attempts to bring alternative agents to market have failed. Sodium channels, which are essential to depolarization and propagation of sensory information in neurons and axons, are a logical target for pain reduction. Attempts to use systemic nonselective sodium channel blockers have not succeeded because of dose-limiting side effects. Now, years of research on sodium channel blockers that are specific to the subtypes located on structures of the peripheral nervous system have come to fruition.2 In this issue of the Journal, Jones and colleagues3 present the results of two clinical trials of VX-548, a novel NaV 1.8 sodium channel blocker to treat postoperative pain, in two commonly used phase 2 clinical models — abdominoplasty and bunionectomy. In the two trials, the sum of the pain-intensity difference (SPID) from baseline to given time points over a 48-hour period was the primary end point. This typical end-point measure in studies involving patients with acute pain allows for evaluation of the treatment response over a clinically relevant period. Both trials showed a reduction in postoperative pain over a period of 48 hours with the highest dose of VX-548, as compared with placebo. Although the trials were not de-signed to detect differences between VX-548 and the control drug, hydrocodone bitartrate–acetaminophen, the results suggest that the selective sodium channel blocker outperformed hydrocodone bitartrate–acetaminophen with respect to pain reduction, side effects, and the dropout rate. At first glance, one could conclude that the differences between VX-548 and placebo were small and not clinically meaningful. Studies have suggested that a clinically meaningful reduction in pain is at least a 2-point improvement or a 30% reduction in the score on an 11-point Likert scale (with scores ranging from 0 [no pain] to 10 [worst possible pain]). These studies either involved patients who had chronic pain after repeated doses of medication or they were singledose studies of breakthrough pain in patients with cancer.4,5 Nevertheless, in studies using the SPID as an end-point measure, clinically meaningful changes have not been clearly established. The current trials showed that a greater percentage of participants who received the highest dose of VX-548 had at least a 30% reduction in the score on the Numeric Pain Rating Scale (NPRS, with scores ranging from 0 to 10, and higher scores indicating a greater level of pain) at 48 hours after the first dose than those who received placebo. However, definitive statements about this threshold of pain reduction are limited because there was no correction for multiple comparisons of this and other secondary end points, and the trial was not powered to detect a between-group difference for this secondary end point. In addition, one may argue that given the high proportion of female participants in these trials (76 to 100% of the participants in each group), the results may not translate to men. Although sex-specific differences in analgesic response have been suggested in studies of chronic pain, sex has consistently been shown to make little difference in the response to oral analgesics in persons with acute pain.6,7 Although the results of these two small phase 2 trials are promising, some concerns need to be addressed. First, ibuprofen, which can be effective in reducing postoperative pain, was allowed as rescue medication, and data on the frequency of use of this drug in the trial groups were not presented. Second, as mentioned above, there is little information regarding comparisons between VX-548 and hydrocodone bitartrate–acetaminophen. More information on the SPID over a period of 4 hours in the VX-548 and hydrocodone bitartrate–acetaminophen groups would be very helpful in understanding the effects of the sodium channel blocker because the pharmacokinetic characteristics of these agents are different; only the differences between VX-548 and placebo over 4 hours are shown graphically in the article. Although VX-548 has a long duration of action, it appears that the onset of pain reduction may be delayed, which would make it less desirable for the treatment of acute pain, although this could be circumvented by using the loading dose evaluated in these trials. In contrast, hydrocodone bitartrate–acetaminophen has a rapid onset of action, but it is a very shortacting agent, and doses every 6 hours may not be adequate to make a fair comparison with VX-548. Third, the SPID measure uses the NPRS, which is a unidimensional patient rating of pain. Patients consider pain relief to consist of a reduction in pain intensity as well as improvement in other domains of pain such as physical functioning and sleep disruption; these domains may be as important as simply numerical pain relief in trials in which repeated doses of medication are used. We are disadvantaged in general because in trials involving patients with acute pain, the best primary end point remains uncertain.7,8 It is perhaps disappointing that the effect size of this very original selective peripheral sodium channel blocker was small, and limited conclusions can be made about its effectiveness as compared with other agents because it was not directly compared with hydrocodone bitartrate–acetaminophen, which is a standard drug for the treatment of acute pain. However, these trials represent an early foray into an exciting new class of drugs in a difficult field. Many early-phase trials of drugs for patients with acute pain are failing to achieve significant results with respect to primary end points despite showing promising positive trends in both primary and secondary end points, including very minimal safety concerns. Sodium channel modulation is one of many mechanisms involved in pain transmission, and it is perhaps unlikely that modulating just one mechanism will lead to large effects on pain. At the moment, postoperative pain is still best managed by multimodal therapies, such as those that combine drugs with different mechanisms. Perhaps our expectations are too high in trials of new agents for acute pain.