Detail
M. Grace Russell Prof. Dr. Timothy F. Jamison
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA, 02139 USA
First published: 09 April 2019
Abstract
Herein, the blockbuster antibacterial drug linezolid is synthesized from simple starting blocks by a convergent continuous flow sequence involving seven (7) chemical transformations. This is the highest total number of distinct reaction steps ever performed in continuous flow without conducting solvent exchanges or intermediate purification. Linezolid was obtained in 73 % isolated yield in a total residence time of 27 minutes, corresponding to a throughput of 816 mg h−1.
The adoption of continuous flow technology1 for the synthesis of natural products,2 active pharmaceutical ingredients (APIs),3 functional materials,4 and other molecules of interest5 has been largely motivated by its key advantages6 relative to batch methodologies. Among these advantages are the ability to access underutilized chemical transformations,7 improve the selectivity of target molecules through greater control of reaction conditions,8 and achieve faster scale‐up and greater synthetic efficiency.9 While a continuous flow strategy offers many benefits, it is interesting to note that a majority of reported syntheses do not exceed two steps and those that do, typically require off‐line intermediate purification. To illustrate, we performed a literature survey by searching the key words “continuous flow” in SciFinder. During the period of January 2018 to October 2018 we located 190 examples of fully‐continuous flow syntheses and 96 % of these were one or two steps. Often, the salient challenges in demonstrating longer continuous reaction sequences (≥3 steps) are the proper management of solvent compatibilities as well as byproduct and side‐product formation. Such challenges have been overcome in several elegant four‐10 and five‐step11 continuous flow syntheses, and in the six‐step continuous flow synthesis of ciprofloxacin we recently reported.12 Herein, we further demonstrate the capabilities of flow through a seven step continuous flow synthesis of linezolid (1) (Figure 1). To the best of our knowledge, this is the highest total number of chemical transformations performed in any continuous flow synthesis reported to‐date that is void of solvent exchanges or intermediate purification.
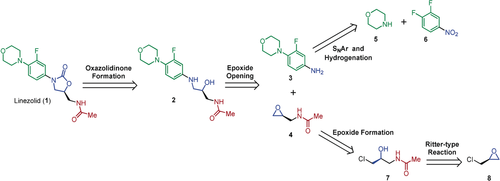
Synthesis planning for linezolid (1). In the synthetic strategy proposed for 1, epoxide 4 was to be accessed through a Ritter‐type reaction of (+)‐epichlorohydrin (8) followed by ring closure of amide 7. Synthesis and coupling of aniline 3 with 4 followed by oxazolidinone formation would generate the target API.
Linezolid (1) (trade name Zyvox, Pfizer) is an antibiotic that is on the World Health Organization's list of essential medicines (Figure 1).13 This API is primarily used as a last line of defense treatment against multi‐drug resistant gram‐positive bacteria. While the reported synthetic routes to linezolid are lengthy (>7 steps) or rely on protecting group manipulations,14 we envisioned a convergent, protecting group‐free synthesis that avoids purification steps between any of the transformations. As shown in Figure 1, our approach was to incorporate epoxide 4 through a regioselective ring‐opening reaction with aniline 3. Following late‐stage oxazolidinone formation of alcohol 2, linezolid (1) would be obtained. Although 4 has previously been proposed as an intermediate in the synthesis of linezolid,14c its synthetic utility was limited due to competitive intramolecular cyclization leading to oxazoline formation. We postulated that the judicious choice of reagents in tandem with the precise control of reaction conditions enabled by a continuous flow strategy such as temperature, stoichiometry, and reaction time, would allow us to successfully engage 4. In this manner, an efficient synthesis of 1 could be achieved.
We began our investigation into the synthesis of linezolid (1) with the preparation of amide 7 by a Ritter‐type reaction between (+)‐epichlorohydrin (8) and acetonitrile (Figure 2). Inspired by previous reports,15 we employed BF3⋅OEt2 as a stoichiometric Lewis acid and allowed the reaction to proceed for a five minute residence time at room temperature. Upon performing an aqueous NaHCO3 quench and purification under batch conditions, amide 7 was obtained in an average of 62 % yield. Although competitive oxazoline 9 formation was largely suppressed under these conditions, appreciable amount of 10 was obtained (ca. 15 %), suggesting the participation of diethyl ether from the Lewis acid as a nucleophile.16 As an effort to eliminate this side‐product formation, we investigated the use of different boron trifluoride etherate complexes with increased steric effects. We were pleased to find that using BF3⋅OBu2 (1.1 equiv) significantly improved the isolated yield of 7 to 90 % under otherwise identical conditions (Figure 2).
.jpg)
Optimization of the continuous flow synthesis of amide 7 from (+)‐epichlorohydrin (8). The yield of amide 7 significantly improved in the presence of excess BF3⋅OBu2 relative to BF3⋅OEt2. tR, residence time.
Having realized reaction conditions to access 7, we attempted to integrate the aqueous quench into the continuous flow sequence by the use of a T‐mixer, but observed rapid clogging of the reactor tubing due to the precipitation of the boric acid. As shown in Figure 3, this was remedied by quenching the Lewis acid with 2‐propanol at −35 °C for 1.2 minutes to generate the soluble boronic ester by‐product (reactor II, Zone 1). Under these conditions, intermediate nitrillium ion 11 also reacted with 2‐propanol to provide imidate 12 as confirmed by 1H‐NMR studies. Epoxide 13 was then formed by treating the stream of 12 with lithium tert‐butoxide in a 1:1 mixture of THF:1,2‐dichloroethane at −35 °C for a residence time of 4 minutes (reactor III, Zone 1).14c The 1,2‐dichloroethane solvent solubilized the lithium chloride salts generated during the course of the reaction and was compatible with the use of the cold reaction temperature (melting point −35 °C). Overall, epoxide 13 featuring a masked amine, was formed within a residence time of 10.2 minutes starting from (+)‐epichlorohydrin (8). It is important to note that variations in the reaction conditions within reactors II and III, namely the equivalents of 2‐propanol and lithium tert‐butoxide, respectively, as well as variations in the cold bath temperatures and residence times, led to significant decreases in the yield of amino alcohol 2 (reactor VI, Zone 3) as monitored by HPLC versus an internal standard. Additionally, by masking the reactive amide group of 12 as an imidate, side‐product formation was minimized in the epoxide‐forming reaction performed in reactor III.14c
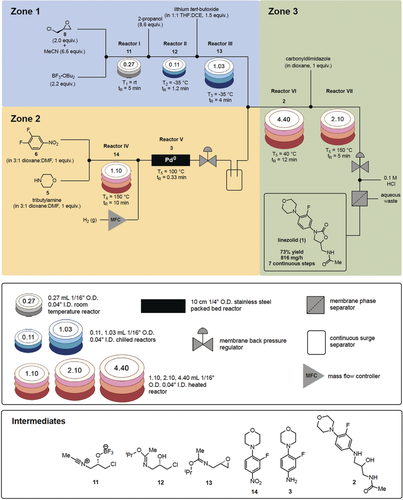
Seven‐step, continuous flow synthesis of linezolid (1). The continuous flow process is visualized as three main zones. Zone 1 generates epoxide 13 through three chemical transformations starting from 8. Aniline 3 is simultaneously generated in Zone 2 from 5 and 6 through two chemical reactions. Finally, the synthesis converges in Zone 3 with the coupling of 3 and 13, followed by oxazolidinone formation. DCE, 1,2‐dichloroethane; DMF, N,N‐dimethylformamide; THF, tetrahydrofuran; tR, residence time; I.D., inner diameter; O.D., outer diameter.
With access to epoxide 13, focus was next directed to the preparation of aniline 3 by a nucleophilic aromatic substitution (SNAr) reaction (reactor IV)17 followed by hydrogenation (reactor V) as shown in Zone 2 of Figure 3. We found that the SNAr reaction between morpholine 5 and 3,4‐dinitrofluoronitrobenzene (6) to form 14 required 10 minutes at 150 °C to proceed to completion in a mixed solvent system comprised of 1,4‐dioxane and N,N‐dimethylformamide. Next, the resulting crude reaction mixture was reduced by introducing hydrogen gas by a mass flow controller (hydrogen gas: liquid flow rate, 15:1) and passing through a compact stainless steel packed bed of palladium(0) at 100 °C and 100 psi of back‐pressure.18 After a residence time of only 0.33 minutes, intermediate 14 underwent complete conversion to aniline 3 and no dehalogenation was observed. The 1,4‐dioxane‐N,N‐dimethylformamide solvent system chosen for this telescoped sequence enhanced the rate of the SNAr, solubilized all starting materials and byproducts, and ensured good compatibility with the palladium packed bed.
Following hydrogenation, the excess hydrogen gas was removed through the use of gravity in a sealed container and the resulting scrubbed reaction stream containing 3 was immediately reintroduced to the system with an additional pump and reacted with epoxide 13 (Figure 3, Zone 3).19 Compound 2 was prepared in 12 minutes at 40 °C in reactor VI without any additional activating agent. We hypothesize that the imidate is hydrolyzed to the corresponding amide in this step, by the water produced from the hydrogenation process in Zone 2. Attempts to increase temperature and decrease the residence time resulted in diminished conversion. It should also be noted that amino alcohol 2 and aniline 3 could not be isolated from the crude reaction mixtures because of rapid oxidation. By utilizing continuous flow technology and designing the synthesis in a manner that enabled it to be fully continuous, deleterious oxidation was minimized through the rapid consumption of these intermediates.
The final oxazolidinone ring forming reaction to generate linezolid (1) was performed in reactor VII by treating the stream of 2 to a solution of N,N‐carbonyldiimidazole in dioxane at 150 °C for five minutes of residence time (Figure 3, Zone 3). Other coupling agents such as phosgene derivatives did not provide 1 presumably due to reaction with nucleophilic components in the crude mixture such as 2‐propanol. After the addition of HCl and in‐line separation, the product was collected and purified off‐line. Overall, linezolid (1) was synthesized in 73 % isolated yield, corresponding to a throughput of 816 mg h−1. HPLC analysis confirmed that the enantio‐enrichment of the product was conserved throughout the entire sequence.
In summary, we have developed an efficient synthesis of linezolid (1). The total residence time for the seven‐step sequence is 27 minutes, which is significantly shorter than reported batch procedures (>60 hours).14c The E‐factor calculated for this process is 25 (an average of 3.57 per chemical transformation). For comparison, industrial pharmaceutical production processes typically attain E‐factors between 25–100.20 Notably, to the best of our knowledge, we achieve the highest number of chemical transformations in a fully‐continuous manner without solvent exchanges, or interruptions for intermediate work‐ups or purifications. These achievements were possible through thoughtful planning of the reaction sequence in a manner that benefitted from the attributes of flow as well as by choosing reagents and solvents that had downstream compatibility and led to minimal byproduct and side‐product formation.
Source: https://doi.org/10.1002/anie.201901814